Unité de Catalyse et de Chimie du Solide site Artois
Faculté des Sciences de Lens
PUBLICATIONS MARQUANTES 2011
:
- 5 publications [à Facteur d'impact > 5] dont 1 couverture
AVEC COUVERTURE- «Unusual Inversion Phenomenon of β-Cyclodextrin Dimers in Water»
S. Menuel, N. Azaroual, D. Landy, N. Six, F. Hapiot, E. Monflier
Chemistry - A European Journal, 2011, 3949-3955 - doi : 10.1002/chem.201003221 - IF = 5,5
 A conformational analysis of three triazole-containing bridged bis-b-cyclodextrins (CD) has been carried out to evaluate their recognition ability. NMR spectroscopy and ITC measurements clearly demonstrate that one of the CD glucopyranose units undergoes a 3608 rotation in water so that the spacer linking the two CDs is deeply included into one of the CD cavities. The amplitude of this inversion phenomenon depends on the nature of the spacer and results in a limited accessibility to the CD cavities in line with previous catalytic results. A conformational analysis of three triazole-containing bridged bis-b-cyclodextrins (CD) has been carried out to evaluate their recognition ability. NMR spectroscopy and ITC measurements clearly demonstrate that one of the CD glucopyranose units undergoes a 3608 rotation in water so that the spacer linking the two CDs is deeply included into one of the CD cavities. The amplitude of this inversion phenomenon depends on the nature of the spacer and results in a limited accessibility to the CD cavities in line with previous catalytic results. |

- «β-Cyclodextrin for design of alumina supported cobalt catalysts efficient in Fischer-Tropsch synthesis»
A. Jean-Marie, A. Griboval-Constant, A.Y. Khodakov, E. Monflier, F. Diehl
Chem. Commun., 2011, 47, 10767–10769 - doi : 10.1039/C1CC13800F - IF = 5,8
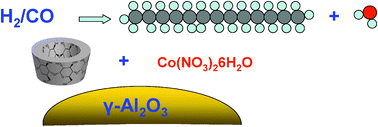 |
| Addition of β-cyclodextrin during catalyst preparation strongly affects the structure and catalytic performance of alumina supported cobalt catalysts for Fischer–Tropsch synthesis. Impregnation of the support with solutions containing β-cyclodextrin leads to higher metal dispersion and spectacularly enhances both reaction rate and heavy hydrocarbons productivity in Fischer–Tropsch synthesis.. |

- «Direct Experimental Evidence for the High Chemical Reactivity of α- and β-Xylopyranosides Adopting a2,5 B Conformation in Glycosyl Transfer»
L. Amorim, F. Marcelo, C. Rousseau, L. Nieto, J. Jimenez-Barbero, J. Marrot,
A. P. Rauter, M. Sollogoub, M. Bols and Y. Blériot
Chem-Eur J 2011, 17, 7345 – 7356 - doi : 10.1002/chem.201003251 - IF = 5,5
| |
| The effect of a2,5B boat conformation on xyloside reactivity has been investigated by studying the hydrolysis and glycosylation of a series of synthetic xyloside analogues based on a 2-oxabicyclo[2.2.2]octane framework, which forces the xylose analogue to adopt a2,5B conformation. The locked β-xylosides were found to hydrolyze 100–1200 times faster than methyl β-D-xylopyranoside, whereas the locked α-xylosides hydrolyzed up to 2×104 times faster than methyl α-D-xylopyranoside. A significant rate enhancement was also observed for the glycosylation reaction. The high reactivity of these conformers can be related to the imposition of a2,5 B conformation, which approximates a transition state (TS) boat conformation. In this way, the energy penalty required to go from the chair to the TS conformation is already paid. These results parallel and support the observation that the GH-11 xylanase family force their substrate to adopt a2,5 B conformation to achieve highly efficient enzymatic glycosidic bond hydrolysis. |

- «Synthesis, Rhodium Complexes and Catalytic Applications of a New Water-Soluble Triphenylphosphane-Modified b-Cyclodextrin»
F.X. Legrand, N. Six, C. Slomianny, H. Bricout, S. Tilloy, E. Monflier
Adv. Synth. Catal. 2011, 353, 1325 – 1334 - doi : 10.1002/adsc.201000917 - IF = 5,2
| |
| A new triphenylphosphane based on a β-cyclodextrin skeleton (PM-β-CD-OTPP) was synthesized. This ligand can be dispersed in water by using the nanoprecipitation method. Transmission electron microscopy and NMR spectroscopy showed that PM-β-CD-OTPP is aggregated in water and forms a stable dispersion. Its aqueous solubility can be dramatically increased in the presence of selected water-soluble guests by formation of inclusion complexes. Associated to a rhodium precursor, PM-β-CD-OTPP is able to generate soluble rhodium species in water. In addition, NMR experiments showed that the cyclodextrin cavity remains accessible for a guest even when PM-β-CD-OTPP is coordinated to rhodium. Finally, this ligand was efficient for rhodium-catalyzed hydrogenation and hydroformylation performed in aqueous medium. |

- «Scope and limitation of activated carbons in aqueous organometallic catalysis»
N. Kania, N. Gokulakrishnan , B. Léger, S. Fourmentin, E. Monflier, A. Ponchel
J. Catal. 2011, 278, 208–218 - doi : 10.1016/j.jcat.2010.12.005 - IF = 5,4
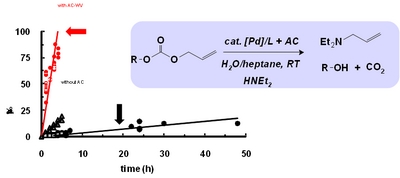 |
| Addition of activated carbon, with appropriate physicochemical characteristics, appears as a simple and efficient method to solve mass-transfer limitations in aqueous organometallic catalysis. New insights into the role of carbon support during the catalytic course are reported. |

Faculté Jean Perrin - rue Jean Souvraz - SP 18 - 62307 Lens Cedex
tel : 03 21 79 17 05
fax : 03 21 79 17 55 |